Degree of Freedom
Introduction: We know that every molecule can move in space in three dimension i.e x , y , z axis. This coordinates are enough to specify it's location in a space. If a molecule is restricted to move in a plane then two coordinates are enough to specify it's location. Let us consider a body which can move in only two direction in a plane i,e x and y direction of cartesian coordinate system, and if it is in motion then its velocity component in x and y direction ( vx and vy)is enough to specify its position and motion. Thus the number of direction in which molecule can move and number of motion it can perform relates the degree of freedom. In the above fig, a molecule can move in translatory direction and can perform rotational motion. This parameter is enough to describe its position and motion
Degree of Freedom : It is defined as total numbers of coordinates or independent quantities required to describe the position and configuration of a system completely.
For monoatomic gases,
Let us consider Helium (He).It can move in three direction in a space i.e in x , y, z direction. Thus it has only 3 degree of freedom.
For diatomic gases,
Let us consider a diatomic molecule like Oxygen O2 or Nitrogen N2 ,
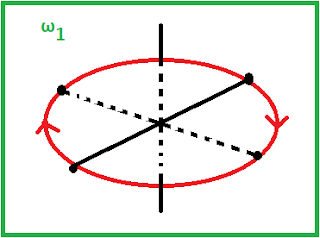
It is free to move in 3 translation direction i.e in x , y, z direction. In additional it is free to perform rotational motion around Y and Z direction, if molecule is situated on X axis. As it is situated on X axis it cannot rotate around X axis in the sense there will be no change in position of molecule while rotating on X axis.
Transalation degree of freedom: 3
Rotational degree of freedom :2
Degree of freedom : 5
If ω1 and ω2 are the angular velocities along Y and Z axis and I1 and I2 are corresponding moment of inertia then total energy is given by,
Total Energy = Etr+ Ero
Etr+ Ero = 1/2 mv2x + 1/2 mv2y + 1/2 mv2z+ 1/2 I1ω21 + 1/2 I1ω22
Molecules like, CO have additional vibrational motion,Thus
Total Energy = Etr+ Ero + Evr
Evr= 1/2 (dy/dt)2 + 1/2(ky)2
where k is force constant and y is vibrational coordinate.
Important note:E ach translation and rotational motion constitute 1/2 Kb T of energy as it have only one squared term but vibrational frequency contributes
Evr = 2 x 1/2 Kb T = Kb T
Be a winner : Every individual is a precious gift by god. Every individual is bestowed with infinite talent within him/her by nature. The human brain is a storehouse of precious collections and this collections is the source to be a winner. Every individual when born start learning new thing, apply it and try to be a best version of himself/herself.Read More
Change Your life: Every people in this world want to be successful. Each individual is abided by the law of nature. The success of each individual is measured in terms of hard work and consistency. The higher the hard work and consistency , the higher is the rate of success. For being a successful person you(each individual)should constantly work on being a unique you(i.e Being different from others). Read More
The 80/20 principle : In our competitive world, each individual have a choice to live a better life, to have more than other. In order to make up with this demand they work harder and harder. But this technique do not yield the expected results, A student learning for 8 hours may not obtain good grades in exam.Read More
Learn Game Development : Computer as well as mobile Game had been on high demand nowadays and many gaming industries around the world face shortage of developers. Since many years Gaming industries have been taking over the market by proving game for android as well as windows phone. It has been a good source of earning as well as learning the behaviour .Read More








No comments:
Post a Comment