Coulomb's Law
Introduction : When the charges are at rest, it develops an electric field around it. When a test charge is brought in this field, the test charge experience a force. On the other hand , if two or more charges are brought together in an electric field they exerts force on each other. The force of attraction exist between two different charge and force of repulsion exist between two similar charge. The magnitude between the charges is given by some law that we are going to see in this topic.
Try this interesting experiment and understand the behaviour of force between charges.
Loading please wait....
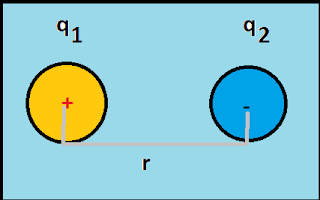 |
| Coulomb's law |
As shown in above fig, there exist two charges q1 and q2 separated by a distance r.
Coulomb's first law : The magnitude of force between two charges is directly proportional to product of both the charges.
Mathematically we can write,
F ∝ q1 . q2 ------(1)
Coulomb's second law : The magnitude of force between two charges is inversely proportional to square of distance between the two charges.
Coulomb's law in vector form
As shown in Fig , q1
and q2
are two
similar point charges situated at points A and B.
r is the distance of separation between them.
FAB denotes the force exerted on q1 by q2 , FBA denotes the force exerted on q2 by q1.
According to coulomb's law force on A due to B is given by ,
According to coulomb's law force on B due to A is given by ,











No comments:
Post a Comment