Atomic Radius
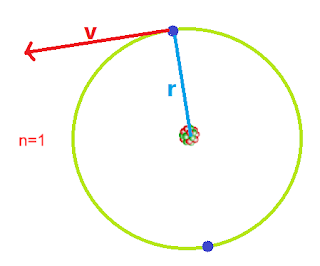
From Bohr's Postulate, a mathematical expression for the radius of Bohr's orbit can be obtained. The distance between the center of the nucleus and the outermost orbit is called an atomic radius.
From Bohr's first postulate
 |
| Bohr's Atomic radius |
Consider an electron of mass m revolving around an atom of radius 'r' with velocity 'v'. Thus the centripetal force required for revolution is provided by the electrostatic force of attraction between the positive nucleus and negatively charged electron. Thus mathematically it can be written as
From Bohr's second postulate:
Equation (C) gives an expression for atomic radius.
where,
h = planck' constant = 6.67 x 10-34
𝛆0 = 8.85x 10-12
n= principal quantum number
m = mass of electron = 9.1 x 10-31
e = elementary charge = 1.6 x 10-19
for n= 1, i.e first orbit
Equ (E) gives the general equation for the radius of an orbit
Related Topics: Atomic Structure Geiger and Marsden Experiment Atomic Spectra Bohr's Postulate Radius of an orbit Velocity of an electron Energy of an orbit Limitation of Bohr's Model De Broglie's explanation Atomic Nucleus Nuclear Binding energy Radioactivity Laws of radioactivity Nuclear energy











No comments:
Post a Comment