The energy of Bohr's orbit
Introduction: According to Bohr's postulate, it is possible to estimate which energy level is allowed by an atom for particular energy of electrons. This postulate is only valid for hydrogen atoms, and ions (for only a single electron system). Let us understand the level of energy allowed for the hydrogen atom.
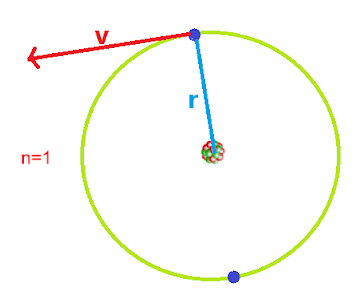 |
| The energy of Bohr's orbit |
From Bohr's first postulate,
1) Kinetic Energy :
2) Potential Energy :
from (A) and ()
Related Topics: Atomic Structure Geiger and Marsden Experiment Atomic Spectra Bohr's Postulate Radius of an orbit Velocity of an electron Energy of an orbit Limitation of Bohr's Model De Broglie's explanation Atomic Nucleus Nuclear Binding energy Radioactivity Laws of radioactivity Nuclear energy













No comments:
Post a Comment